CASCADE® Platelet Rich Fibrin
Membrane (PRFM)
CASCADE® Platelet Rich Fibrin Membrane (PRFM)
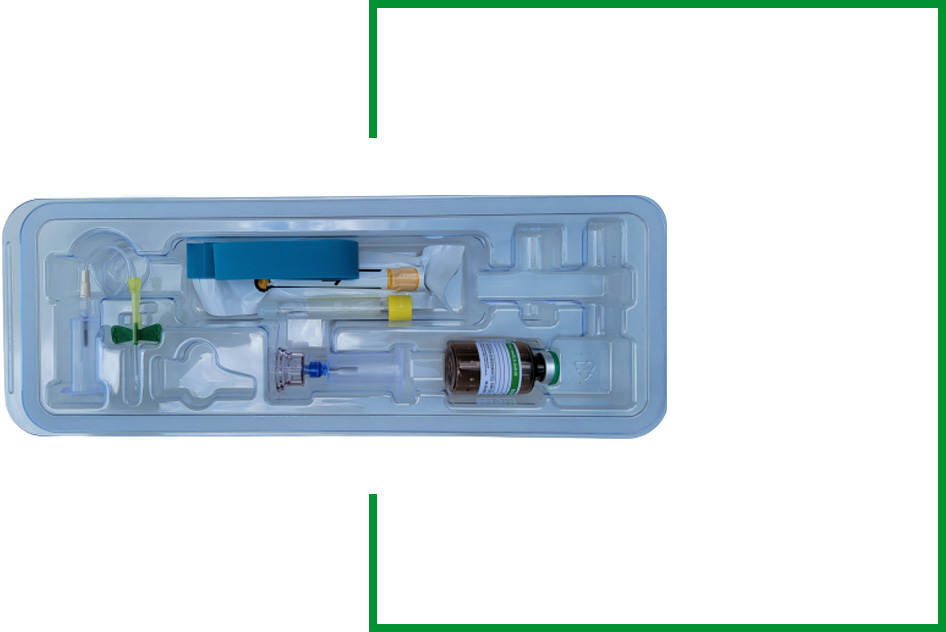
The CASCADE® Platelet Rich Fibrin Membrane (PRFM) is a self-contained disposable kit with all the necessary components to extract and concentrate a patient’s platelets and associated growth from a small sample of blood at point of care.
The body’s own capacity to stimulate tissue healing can vary based on access to critical growth factors (GF). Circulating platelets have a half-life of 7-10 days and secrete GFs (1000+) which have a variety of beneficial functions including anti-inflammatory, anti-infection, cell recruitment, and more. In recent years, physicians are using novel technologies to extract and concentrate autologous platelets and their associated growth factors to potentially stimulate areas with limited vascular supply, hard-to-heal and/or non-healing injuries, revision procedures, and for high-risk patient populations.1
Top Value Proposition:
The CASCADE® PRFM kit solves the inherent challenge of delivering concentrated platelets to a wound bed or surgical site without being washed away and maintaining the viability of the platelets for >7 days.2 Many platelet systems use exogenous thrombin (e.g., bovine or human) to gel or clot the concentrated platelets at the site, however, studies show an immediate bolus response activating platelets and releasing all the associated growth factors at once.3,4 The CASCADE® PRFM converts plasma (e.g., fibrinogen), rich in platelets, into a fibrin membrane scaffold without using exogenous thrombin. This scaffold protects the platelets during delivery and placement at the injury site allowing for a sustained release of associated platelet growth factors over time.5
Key Features & Benefits:
- The CASCADE®PRFM kit concentrates platelets and the full array of natural growth factors from the patient’s blood
- Off-the-shelf, sterile, closed system with pre-loaded anti-coagulant blood collection tubes to reduce steps and simplify the process
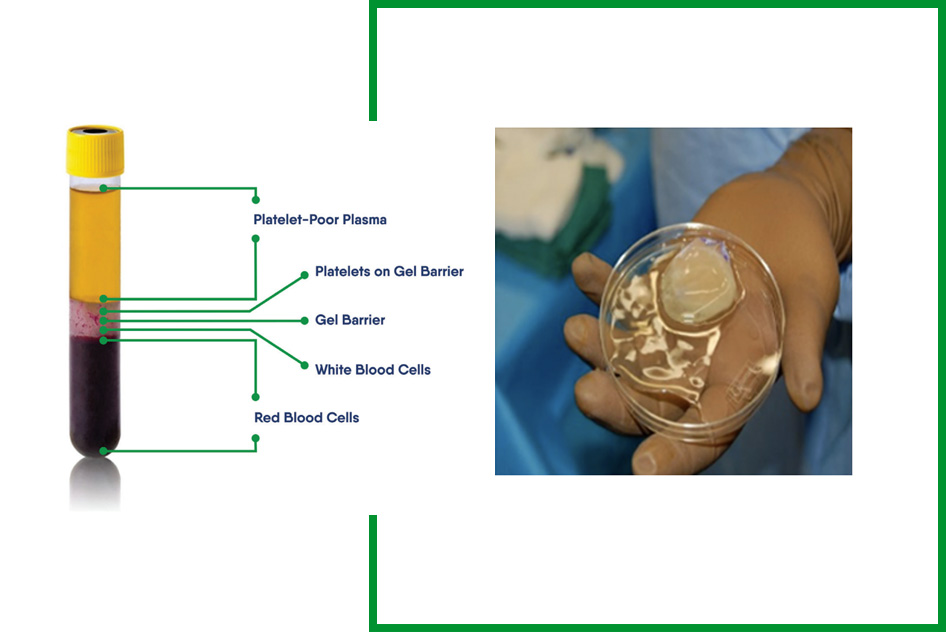
- Double-spin centrifuge technique converts liquid platelets and plasma into a solid membrane
- Six-minute (6 min) spin separates the platelets and plasma from the other blood components using a proprietary separation gel technology (liquid form)
- Twenty-five (25 min) spin converts plasma/fibrinogen into a fibrin scaffold trapping the intact unactivated platelets (solid form)
- ONLY system w/ concentrated platelets & associated growth factors available out to 7 days5
- 510K clearance allows for the ability to mix with autograft or allograft bone per the clinical use requirements
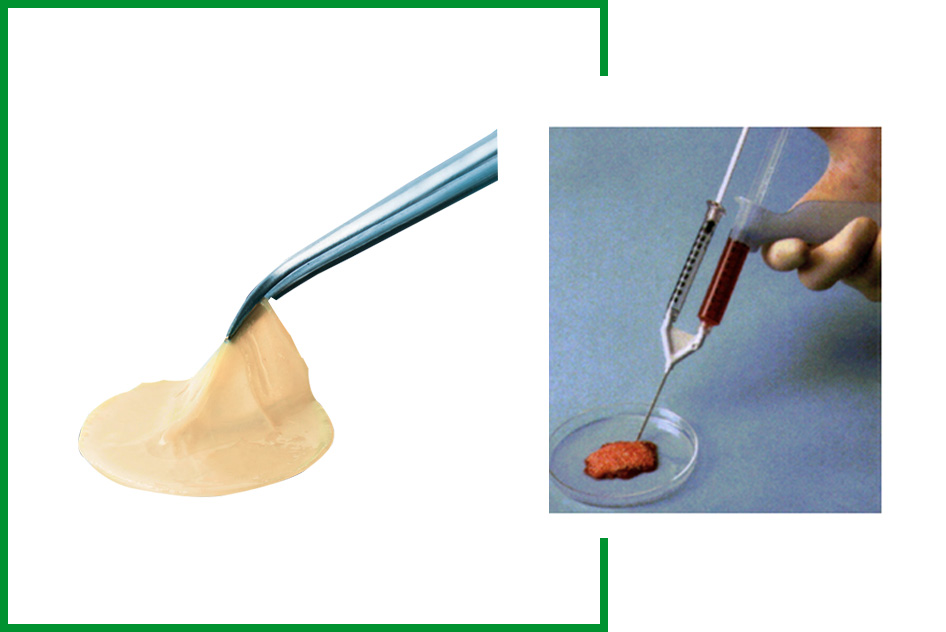
- Six-minute (6 min) spin separates the platelets.
- Twenty-five (25 min) spin converts into a fibrin scaffold.
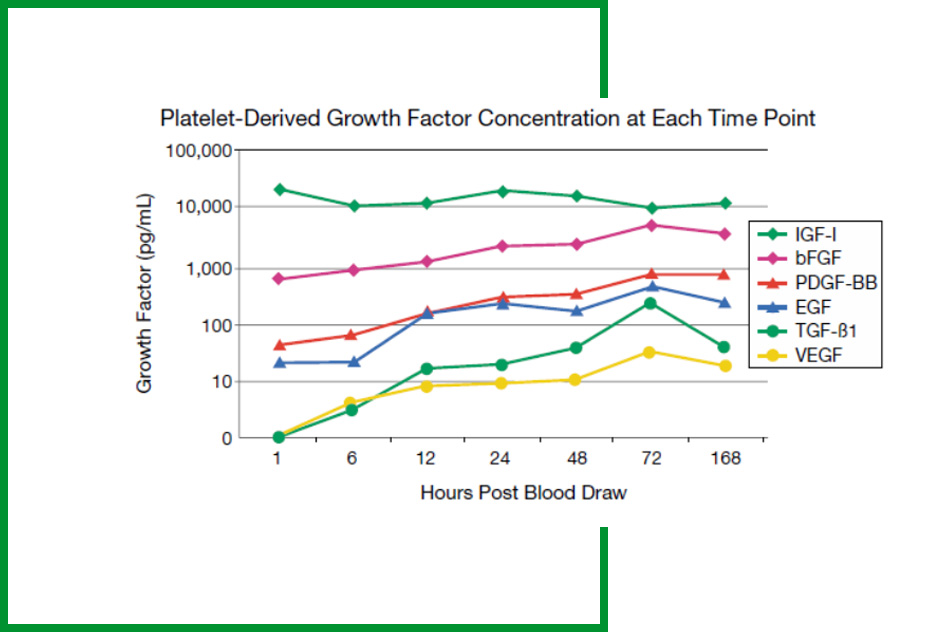
Cascade® releases growth factors into the wound in a coordinated and continuous process in accordance with a natural genetic sequence without the use of platelet activators.
This permits recruitment of the appropriate cell types into the wound at the proper time over 7+ days to initiate tissue regeneration.
FDA 510K#: BK060006
Official Device Name: CASCADE™ Autologous Platelet System
Intended Use:
The CASCADE™ Autologous Platelet System is designed to be used for the safe and rapid preparation of autologous platelet-rich-plasma (PRP) from a small sample of blood at the patient point of care. The PRP can be mixed with autograft and allograft bone prior to application to an orthopedic surgical site as deemed necessary by the clinical use requirements.
CLINICAL STUDIES:
Please review several of our clinical studies showcasing the efficacy of PRP in wound treatment.
- In a single‑center, consecutive series study (50 patients) on the use of a novel platelet‑rich fibrin matrix (PRFM) and beta‑tricalcium phosphate, Cammisa et. al, showed a 92.4% fusion rate at 12 months using CT scans without complications, infections, or revisions. Significant improvement in VAS scores for both back and leg pain (p<0.05) and the number of patients using opioid analgesics at 12-months decreased by 38% in posterolateral lumbar fusion.6
- In a randomized, controlled trial, Barber et. al, used the platelet‑rich fibrin membrane (PRFM) to augment rotator cuff repair surgery by comparing a triple-loaded, single-row repair (two fixation anchors) with PRFM vs. a double-row repair (4 anchors). Both repairs showed excellent results however, the PRFM group used two fewer anchors.7
- A novel autologous platelet-rich fibrin membrane (PRFM) was used to facilitate healing in patients with unresponsive lower-extremity wounds. All patients enrolled in the study had failed conventional treatment, received up to three applications of the PRFM (35 to 50 mm fibrin disc), and were assessed using standard methods. Sixty-seven percent (67%) of the patients healed with an average of two applications in under 8 weeks.8
Click on the links below to download literature
References:
- Anitua E., Andia I. – Thromb. Haemost. 2004; 91: 4-15
- Lucarelli et al., Eur. Cells Mater, 2010
- Han et. al., JBJS, 2009
- Marx RE et.al., Implants Dental 10:225-228, 2001
- Roy et. al., 2011
- Cammisa et. al, European Spine Journal, 2018
- Barber et. al, Journal of Arthroscopy and Related Surgery, 2016
- Dardik et. al Wound Repair and Regeneration, 2008
